What is the difference between serial dilution and dilution factor?
A Serial dilution is a series of dilutions, with the dilution factor staying the same for each step. The concentration factor is the initial volume divided by the final solution volume. The dilution factor is the inverse of the concentration factor.
Why is serial dilution better than simple dilution?
In a series of dilutions, each step has the same fold-dilution factor, creating a consistent geometric process with a constant ratio between every pair of consecutive dilutions. Additionally, serial dilutions permit more precise regulation over the dilution factor.
What is direct dilution?
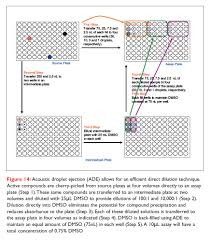
Direct dilution is the process by which Echo Liquid Handlers generates the IC50 curve, transferring precise droplets of solution directly to individual assay wells, without tips.
What is the difference between serial dilution and proportional dilution?
The main difference between serial dilution and proportional dilution is that in serial dilution, the amount of the original solution used for each dilution step is constant, whereas in proportional dilution, the amount of the original solution used for each dilution step varies depending on the desired dilution factor …
What is the difference between direct and serial dilution?
The direct dilution method uses far less sample than the serial dilution method. This figure shows only the first four concentrations via direct dilution. More complete examples covering 12 half-log steps are covered in the literature. Direct dilution follows a simpler process.
What is the difference between linear and serial dilution?
The main difference between the linear and serial dilution is that in serial dilution the dilution factor remains constant but in linear dilution it can change. Eg- 1M to 0.1M to 0.01M to 0.001M, the dilution factor here is 10, thus it is a serial…
Why is serial dilution not used?
Two significant issues confront serial dilution techniques. Error propagation across columns or rows is the first. Transfer imperfections result in less accurate and precise dispensing with each subsequent serial dilution stage. As a result, the largest dilutions will produce the most erroneous results.
What are the disadvantages of serial dilution method?
However, there are also some disadvantages. One concern is the accuracy and overall usefulness of serial dilution tests, as they can lead to significant differences in microbial compositions and raise concerns about the reliability of the results.
What are the advantages of serial dilution?
Serial dilution has many advantages: the materials necessary are typically already present in the lab and require no special engineering. Conditions can be adjusted as the experiment progresses (e.g., drug concentrations increased as drug resistance improves).
Is serial dilution more accurate?
Then 1 mL (aliquot) of the second tube is taken and transferred to the third tube. The process of transferring aliquot is repeated until the desired dilution concentration is achieved. Although serial dilution requires more preparation, it will give more accurate dilutions, provided that your technique is precise.
When to do serial dilution?
Consider When to Use Serial Dilutions: If you need to dilute a protein or chemical stock by 1000-fold or more, it is best to use some serial dilution.
What are the two methods of dilution?
There are 2 main types of dilutions: simple and serial.
Why a serial dilution is preferred for large dilutions?
Serial Dilution Series: Each concentration is created by diluting the previous one. This approach allows for accurate dilutions across a broader concentration range.
What is the main purpose of serial dilution?
A serial dilution is a step-wise series of dilutions, where the dilution factor stays the same for each step. The purpose of a serial dilution is to estimate the concentration of a sample, or to obtain the desired concentration of a reagent, chemical or compound.
What is the difference between serial dilution and simple dilution?
A serial dilution is simply a series of simple dilutions which amplifies the dilution factor quickly beginning with a small initial quantity of material (e.g., DNA, restriction enzyme, etc.). The source of dilution material for each step comes from the diluted material of the previous.
What is a direct dilution?
Direct dilutions The alternative approach to serial dilution is the direct dilution of micro-volumes of compound (ie on a volumetric basis). In this case the volume actually dispensed is directly proportional to the amount of compound required to give the desired concentration in the chosen final assay volume.
What is the difference between serial and parallel dilution?
Serial dilutions are created from a stock solution and the initial solution is diluted. The diluted solution is diluted again and so on, so that all the solutions are from the solution before. Parallel solutions are separate dilutions created from the original stock solution.
What is the advantage of serial dilution over direct dilution when you are preparing a small volume of solution with high dilution factor?
Performing a serial dilution instead of directly diluting various amounts of a stock solution offers key advantages, such as minimizing material waste, being cost-effective, and ensuring precise concentration gradients over a broad range.
What is the main disadvantage of the serial dilution technique?
However, there are also disadvantages associated with serial dilution-agar plate procedure. The primary disadvantage is that the process is time-consuming and requires a high level of skill to perform accurately. Also, cultures grown on agar plates generally don’t survive for long due to dehydration.
What is an advantage of performing a series of dilutions vs. a simple dilution?
Answer and Explanation: Serial dilution is a convenient way to determine the number of cells in an original solution when it is unknown how concentrated that solution is. A single dilution may not be sufficient to bring the concentration to countable levels, thus a series of dilution is preferred.
Does serial dilution decrease concentration?
This means that our dilution factor was ten, and we performed a 10-fold serial dilution. This method is commonly used if you want to get from a very high concentration to a much lower concentration in as few steps as possible.
Is serial dilution more accurate than direct dilution?
So in general direct dilution is preferred to minimize errors. But there some edge cases where serial dilution might be better. For example, if the size differences are extremely large, then it might not be possible to find apparatus with high enough precision.
When should you use the serial dilution technique?
A dilution factor is often used, which indicates the factor by which the stock is diluted. Serial dilutions are commonly used to avoid having to transfer a very small volume to make a highly diluted liquid. This method is commonly used in both chemistry and biology.
Why must must we use a serial dilution and not a direct plate count when trying to determine how many microbes are present in a sample?
We use serial dilutions to create decreasing concentrations of the original sample that are then plated so that a plate will be created with a low enough number of bacteria that we can count individual colonies. From that number, we can calculate the original cell density in the broth.
What is a major advantage of performing a serial dilution?
There are various advantages of using serial dilution in microbiology. Serial dilution helps to gradually decrease a high concentrate cell culture to a more manageable and practical level. Counting the colonies obtained from these samples helps estimate the concentration of an unknown substance.
What are the two main uses of the serial dilution?
Serial two-fold and ten-fold dilutions are commonly used to titer antibodies or prepare diluted analytes (for a standard curve for example). Serial dilutions are also commonly used to avoid having to pipette very small volumes (1-10 µl) to make a dilution of a solution.
What is the dilution factor of a dilution series?
Dilution factor = amount of specimen transferred divided by the total volume after transfer[amount of specimen transferred + amount of diluent already in tube]. Determine the dilution factor for each tube in the dilution series. Multiply the individual dilution factor for the tube and all previous tubes.
What is the difference between dilution factor and dilution ratio?
Dilution ratio refers to a simple dilution, in which a unit volume of a solute is combined with a desired volume of solvent. Dilution factor on the other hand refers to the ratio of the volume of the initial concentrated solute to the total volume of the final diluted solution.
What is dilution factor?
After dilution, the dilution factor (or dilution ratio) represents how much of the original stock solution remains in the entire solution. It’s usually expressed as a ratio, although it can also be expressed as an exponent.
What is the difference between serial dilution and MPN?
An MPN can be computed for any positive number of tubes at any positive number of dilutions, but often serial dilutions use three or more dilutions and a decimal series (Each dilution has one tenth as much of the original sample as the previous dilution.)
What is a serial dilution?
What is the alternative approach to serial dilution?
What is direct dilution?
Are serial dilutions reproducible?
Serial Dilution: A Step-by-Step Approach
Let’s start with serial dilution. This method involves a series of dilutions, each one using the previous diluted solution as the starting point. Think of it like building a staircase: You start at the bottom and take one step at a time until you reach the top.
Here’s how it works:
1. Start with your concentrated stock solution. This is your starting point, the highest concentration you’re working with.
2. Transfer a specific volume of the stock solution into a new container. The volume you transfer will determine the dilution factor.
3. Add a specific volume of the solvent (usually water) to the transferred solution. The solvent dilutes the stock solution, lowering its concentration.
4. Mix the solution thoroughly to ensure the solute is evenly distributed.
5. Repeat steps 2-4 using the diluted solution from the previous step. This is where the “serial” part comes in. You’re essentially taking a portion of your previously diluted solution and diluting it further.
Why choose serial dilution?
Serial dilutions are fantastic for generating a series of solutions with progressively decreasing concentrations. This is particularly useful when you need a wide range of concentrations for experiments or analysis. Imagine you’re testing a drug’s effectiveness at different concentrations: Serial dilution allows you to create those concentrations efficiently.
Direct Dilution: A One-Step Solution
Now, let’s move on to direct dilution. This method involves directly diluting the stock solution to the desired final concentration in a single step. It’s like taking the elevator straight to the top floor, bypassing all the intermediate steps.
Here’s how it works:
1. Start with your concentrated stock solution. Just like with serial dilution, this is your starting point.
2. Calculate the volume of the stock solution and the solvent needed to achieve the desired final concentration and volume.
3. Transfer the calculated volume of the stock solution into a new container.
4. Add the calculated volume of the solvent (usually water) to the stock solution.
5. Mix the solution thoroughly to ensure the solute is evenly distributed.
Why choose direct dilution?
Direct dilution is simpler and faster when you only need a single diluted solution. It’s ideal when you have a specific concentration target in mind and don’t need a range of concentrations.
Serial Dilution vs. Direct Dilution: A Comparative View
Let’s summarize the key differences between serial dilution and direct dilution in a table:
| Feature | Serial Dilution | Direct Dilution |
|————————-|—————————————|————————————–|
| Approach | Multiple dilutions in series | Single dilution |
| Number of Steps | Multiple | One |
| Concentration Range | Wide range of decreasing concentrations | Single, specific concentration |
| Accuracy | More susceptible to errors in each step | More accurate with precise calculations |
| Time Efficiency | Can be time-consuming | Faster and efficient |
| Applications | Experiments requiring a range of concentrations | Specific concentration requirements |
When to Use Each Method
Here’s a quick guide to help you decide when to use serial dilution vs direct dilution:
Choose serial dilution when you need a series of solutions with progressively decreasing concentrations, for example:
Standardization of solutions: Creating a series of dilutions for titration experiments.
Calibration curves: Generating a range of concentrations for spectrophotometry or other analytical techniques.
Drug testing: Determining the efficacy of a drug at different concentrations.
Choose direct dilution when you only need a single diluted solution at a specific concentration, for example:
Preparing reagents for experiments: Diluting a stock solution to the exact concentration required for a particular experiment.
Making solutions for everyday use: Diluting a concentrated cleaning solution for home use.
Understanding Dilution Factors
To understand dilution methods, you need to grasp the concept of dilution factors. The dilution factor represents the ratio of the final volume to the initial volume. It tells you how many times the solution has been diluted.
For example, a dilution factor of 10 means the final volume is 10 times larger than the initial volume. This also means the concentration is 10 times lower than the initial concentration.
Working with Formulas
To calculate dilutions, use the following formula:
C1V1 = C2V2
Where:
C1 = Initial concentration of the stock solution
V1 = Volume of the stock solution taken
C2 = Final concentration of the diluted solution
V2 = Final volume of the diluted solution
Let’s look at an example:
You want to dilute a 10M stock solution to a 0.5M solution with a final volume of 100 mL. Here’s how to calculate the required volume of the stock solution:
1. C1 = 10M
2. V1 = ? (This is what we want to calculate)
3. C2 = 0.5M
4. V2 = 100 mL
Using the formula C1V1 = C2V2:
10M * V1 = 0.5M * 100 mL
V1 = (0.5M * 100 mL) / 10M
V1 = 5 mL
Therefore, you need to take 5 mL of the 10M stock solution and add it to 95 mL of water (100mL – 5mL) to achieve a 0.5M solution with a final volume of 100mL.
Safety Considerations
While serial dilution and direct dilution are simple techniques, safety is paramount, especially when working with concentrated solutions. Always wear appropriate personal protective equipment (PPE) like gloves and safety goggles. Follow these guidelines:
Work in a well-ventilated area. Some solutions can release harmful fumes.
Use clean glassware. Contamination can affect the accuracy of your dilutions.
Label all solutions clearly. Avoid confusion and mistakes.
Dispose of waste properly. Follow your laboratory’s waste disposal guidelines.
FAQs
What is the difference between serial dilution and direct dilution?
Serial dilution involves a series of dilutions, each using the previously diluted solution as the starting point. Direct dilution involves diluting the stock solution to the desired final concentration in a single step.
When should I use serial dilution?
Use serial dilution when you need a series of solutions with progressively decreasing concentrations, for example, in experiments requiring a wide concentration range.
When should I use direct dilution?
Use direct dilution when you only need a single diluted solution at a specific concentration, for example, in preparing reagents for experiments or making solutions for everyday use.
How do I calculate the dilution factor?
The dilution factor is the ratio of the final volume to the initial volume. For example, if you dilute 1 mL of solution to 10 mL, the dilution factor is 10.
What are the advantages and disadvantages of each method?
Serial dilution: Advantages: useful for generating a wide range of concentrations, potentially more accurate for very low concentrations. Disadvantages: time-consuming, susceptible to error accumulation.
Direct dilution: Advantages: quick and simple, less susceptible to error. Disadvantages: only produces a single concentration, less accurate for very low concentrations.
What is the most accurate dilution method?
Direct dilution is generally considered more accurate as long as you have the necessary equipment and expertise to measure volumes precisely.
Can I perform serial dilutions with different dilution factors?
Yes, you can use different dilution factors in each step of serial dilution. This can be useful for generating a specific range of concentrations.
What if I need to make a very dilute solution?
For very dilute solutions, serial dilutions are often more practical and accurate, as they minimize the risk of errors introduced by measuring small volumes.
What are some examples of serial dilutions in real-world applications?
Drug testing: Determining the efficacy of a drug at different concentrations.
Standardization of solutions: Creating a series of dilutions for titration experiments.
Calibration curves: Generating a range of concentrations for spectrophotometry or other analytical techniques.
What are some examples of direct dilutions in real-world applications?
Preparing reagents for experiments: Diluting a stock solution to the exact concentration required for a particular experiment.
Making solutions for everyday use: Diluting a concentrated cleaning solution for home use.
Understanding the differences between serial dilution and direct dilution is crucial for working effectively with solutions. By choosing the right method, you can ensure accurate and efficient dilutions, which are essential for accurate experiments and reliable results.
See more here: Why Is Serial Dilution Better Than Simple Dilution? | Serial Dilution Vs Direct Dilution
Serial vs Direct Dilution – Time to apply new thinking to
The alternative approach to serial dilution is the direct dilution of micro-volumes of compound (ie on a volumetric basis). In this case the volume actually dispensed is directly proportional to the Drug Discovery World (DDW)
How to do serial dilutions (including calculations)
A serial dilution is a step-wise series of dilutions, where the dilution factor stays the same for each step. The purpose of a serial dilution is to estimate the concentration of a sample, or to obtain the INTEGRA Biosciences
1.8: Serial Dilutions and Standard Curve – Biology
A Serial dilution is a series of dilutions, with the dilution factor staying the same for each step. The concentration factor is the Biology LibreTexts
Master the art of pipetting and learn how to perform serial
A serial dilution is the step-wise reduction of a concentration using a constant dilution factor and similar volumes. This highly standardized procedure has applications in drug INTEGRA Biosciences
Serial Dilution: Formula, Calculator, Method, Uses,
Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9 Microbe Notes
Guide: Learn how to perform serial dilutions with INTEGRA
The main advantage of a serial dilution over a direct dilution is the ability to achieve a similar change in concentration with higher transfer volumes. One purpose INTEGRA Biosciences
SERIAL vs DIRECT DILUTION Time to apply new thinking to
A new technique -referred to as ‘direct dilution’ -eliminates traditional serial dilutions and the compounded error that they are prone to experience (7). With ADE, ResearchGate
Reproducibility in Drug Discovery & Development: Direct Dilution
Reproducibility in Drug Discovery & Development: Direct Dilution vs. Serial Dilution. Russ DuChene. Published Sep 27, 2018. + Follow. Eliminate Steps While LinkedIn
Serial Dilution – an overview | ScienceDirect Topics
Serial dilutions (1:2, 1:4, 1:8 etc.) are a possible means of looking for interferences. The simple concept is that in the absence of an interferent, as a sample is progressively ScienceDirect
Serial Dilutions – UMass
Serial dilutions are much easier to make and they cover the range evenly. Serial dilutions are made by making the same dilution step over and over, using the previous dilution as marlin
See more new information: curtislovellmusic.com
Dilution Series \U0026 Serial Dilution
How To Prepare A Serial Dilution
Interpreting An Elisa Using A Serial Dilution
How To Perform Serial Dilutions In Microbiology
Dilution And Dilution Factor In Microbiology|| How To Calculate Dilution Factor In Serial Dilution?
Serial Dilutions | Microbiology
As Biology – How To Calculate Serial And Simple Dilutions
Serial Dilution | Required Practical Revision For Biology And Chemistry A-Level
Link to this article: serial dilution vs direct dilution.
See more articles in the same category here: https://curtislovellmusic.com/category/what/