What did the gold foil experiment using alpha particle scattering conclude?
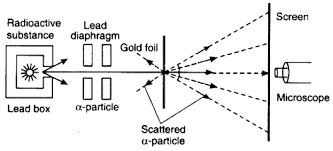
During Rutherford’s gold foil experiment, a beam of alpha particles was directed at a thin sheet of gold foil. This helped to prove the existence of a nucleus in the atom.
What was the conclusion from Rutherford scattering of alpha particles with gold foil?
Conclusions are drawn by Rutherford from his α-ray scattering experiment: α-particles passed through the gold foil without any deflection concluding the empty space inside the atom. Deflection is observed in a few particles which proves the positive charge of the atom occupies very little space.
What was proved by Rutherford alpha particle scattering experiment?
Rutherford’s α – particle scattering experiment showed that the mass and positive charge of the atom is concentrated in the nucleus and most of the space in the atom is empty.
What did Rutherford conclude from the alpha particle scattering experiment except?
Rutherford’s α-particle scattering experiment concluded that all the positive charge inside an atom is concentrated only on a small part inside the nucleus which is present at the centre of the atom along with the neutrons.
What happened in Rutherford’s gold foil experiment?
Rutherford’s gold foil experiment showed that atoms are mostly empty space, with the positive charge concentrated in a nucleus. He realized this because most of the alpha particles passed straight through the piece of gold foil, with just a few deflected at huge angles.
Why did Rutherford use gold foil in his alpha particle scattering experiment?
Rutherford needed a metal sheet that could be as thin as feasible for the scattering experiment. Gold is the most malleable metal on the planet. It is simple to make very thin sheets out of it. Thus, Rutherford chose gold foil for his alpha-ray scattering experiment.
What major conclusions were made from Rutherford’s gold foil experiment?
Rutherford’s gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus.
How could you explain the alpha particles being repelled by the gold foil?
Alpha particles are positively charges. Therefore, when some of them would come in contact with the positive nuclei of the gold atoms they would be repelled back toward their source.
What were the results of the alpha scattering experiment?
Most alpha particles went straight through the foil. But a few were scattered in different directions. This evidence led Rutherford to suggest a new model for the atom, called the nuclear model close nuclear modelThe scientific idea that an atom has electrons surrounding a nucleus that contains protons and neutrons..
What did scientists expect to see during the alpha particle scattering experiments?
The atom at the time was believed to have been a dense clump of positive and negative particles (protons and electrons). This was called the ‘Plum Pudding’ model. Thus it was expected that if we fired positively charged particles at gold foil (a sheet of atoms) they would rebound back.
How thick was the gold foil used by Rutherford?
The gold foil was only 0.00004 cm thick. Most of the alpha particles went straight through the foil, but some were deflected by the foil and hit a spot on a screen placed off to one side. Geiger and Marsden found that about one in 20,000 alpha particles had been deflected 45° or more.
What was the conclusion of Rutherford alpha particle scattering?
His experiments proved that the atom is largely empty and has a heavy positive-charged body at the center called the nucleus. The central nucleus is positively charged and the negatively-charged electrons revolve around the nucleus.
What did Rutherford conclude about alpha particles?
Rutherford and coworkers were able to demonstrate that the alpha particle was an atom of helium (later to be determined to be a nucleus of helium), and that helium gas would accumulate or be entrapped in minerals that contained radium.
Which of the following conclusions of Rutherford’s experiment is true?
Rutherford’s α-particle scattering experiment gives the experimental evidence for deriving the conclusion that most of the space inside the atom is empty.
What did the Rutherford alpha particle experiment prove?
Rutherford’s experiments on the scattering of alpha particles proved that the atom is mostly empty. The whole mass and positive charge of atoms are concentrated in a very small region at the center known as the nucleus. The positive charge on the nucleus is due to protons.
What did Rutherford’s model explain?
According to Rutherford model, an atom consists of positively charged particles concentrated at the center known as the nucleus. The size of the nucleus is very small as compared to the size of the atom. The electrons revolve around the nucleus in well-defined orbits. Most of the space inside an atom is free.
What were the expectations of the gold foil experiment?
The alpha particles would pass through the gold foil with some slight deflections.
What did Rutherford’s gold foil experiment tell us?
Rutherford’s gold foil experiments (he also used other metal foils) showed that the atom is mostly empty space with a comparatively tiny, massive, positively charged nucleus in the center.
Why did the Rutherford model fail?
The reason for the failure of Rutherford’s atomic model was that it could not account for the stability of an atom.
What would happen if Rutherford used aluminum foil?
The repulsion between alpha particles and heavy nuclei is much larger than the repulsion between alpha particles and light nuclei. So if a lighter element like aluminium is used instead of gold, the number of alpha particles deflected will be much less in comparison to the deflections observed when gold was used.
What was the major outcome of Rutherford’s gold foil experiment?
A piece of gold foil was hit with alpha particles, which have a positive charge. Most alpha particles went right through. This showed that the gold atoms were mostly empty space.
Why did Rutherford choose gold foil?
Rutherford used gold for his scattering experiment because gold is the most malleable metal and he wanted the thinnest layer of metal. The gold sheet used was around 1000 atoms thick. Therefore, Rutherford selected a gold foil in his alpha scattering experiment.
Do alpha particles have a positive charge?
A positively charged particle ejected spontaneously from the nuclei of some radioactive elements. It is identical to a helium nucleus that has a mass number of 4 and an electrostatic charge of +2.
What was the conclusion of the alpha particle gold foil experiment?
The space inside an atom is empty as most of the α-particles passed without deflection through the gold foil. The positive charge occupies a minimum space which indicates that very few particles were diverted from their path.
What is the scattering of alpha particles by gold foil?
Scattering of a-particles by a thin gold foil suggests the presence of a positively charged nucleus at the centre of an atom. The experiment was conducted by Ernest Rutherford. He also discovered the Proton.
Why were alpha particles deflected in Rutherford’s experiment?
Reason: The positive charge of the atom is spread throughout the atom that repelled and deflected the positively charged α-particles.
What conclusion was drawn from the gold foil experiment?
Rutherford’s gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Based on these results, Rutherford proposed the nuclear model of the atom.
What did most of the alpha particles do in the gold foil experiment?
Most alpha particles passed straight through the gold foil, which implied that atoms are mostly composed of open space. Some alpha particles were deflected slightly, suggesting interactions with other positively charged particles within the atom.
What did the gold foil experiment alpha particle scattering prove GCSE?
the fact that most alpha particles went straight through the foil is evidence for the atom being mostly empty space.
What happens when the charged particles strike the surface of the gold foil?
However, due to the positively charged protons which are located in the nucleus at the center of the atom, a very small percentage (about 1 in 8000 particles) bounced off the gold foil at very large angles. Some were even redirected back toward the source.
What was Rutherford’s gold foil experiment?
How did Rutherford find out if alpha particles were deflected?
What were the Rutherford scattering experiments?
What did Rutherford discover?
So, back in the early 1900s, scientists were just starting to understand what atoms were made of. The prevailing model was called the plum pudding model, which basically said that atoms were like a ball of positive charge with negative electrons scattered throughout, kind of like plums in a pudding.
Ernest Rutherford wasn’t buying it. He wanted to get a closer look at the atom, and he thought a good way to do that was to bombard a thin sheet of gold foil with alpha particles, which are basically positively charged helium nuclei. He reasoned that if the plum pudding model was right, the alpha particles should pass right through the gold foil with only a slight deflection, kind of like a bullet going through a jelly.
But that’s not what happened.
What Rutherford discovered was mind-blowing.
Most of the alpha particles did pass through the gold foil with little to no deflection, which was kind of expected. But some of them were deflected at very large angles, and some even bounced right back!
Rutherford’s experiment concluded that:
The atom is mostly empty space. This is because most of the alpha particles passed straight through the gold foil.
The atom has a tiny, dense, positively charged nucleus. This is because a few of the alpha particles were deflected at very large angles, suggesting they were hitting something very dense and positively charged.
The nucleus is responsible for most of the atom’s mass. This is because the nucleus contains almost all of the atom’s protons and neutrons.
This discovery completely overturned the plum pudding model and led to the development of the nuclear model of the atom. In this model, the atom consists of a small, dense nucleus at the center, which contains the protons and neutrons, and a cloud of negatively charged electrons orbiting the nucleus.
Think of it like this: Imagine the nucleus as a tiny marble in the middle of a football field. The electrons are like tiny flies buzzing around the marble. Most of the space inside the atom is empty.
Rutherford’s experiment was a major breakthrough in our understanding of the atom, and it paved the way for further discoveries in nuclear physics.
FAQs
Q: Why did Rutherford choose gold foil?
A: Gold is a very malleable metal, which means it can be hammered into very thin sheets. This allowed Rutherford to create a thin enough sheet of gold for the alpha particles to pass through.
Q: What were the limitations of Rutherford’s experiment?
A: Rutherford’s experiment was a groundbreaking experiment, but it had some limitations. For example, it didn’t tell us anything about the arrangement of electrons around the nucleus. It also didn’t tell us about the existence of neutrons, which were discovered later.
Q: How did Rutherford’s experiment change our understanding of the atom?
A: Rutherford’s experiment revolutionized our understanding of the atom. It showed that the atom is not a solid, indivisible sphere, but rather a complex structure with a tiny, dense nucleus at the center and a cloud of electrons orbiting around it. This understanding paved the way for the development of modern atomic theory.
Q: What other experiments were done after Rutherford’s experiment?
A: After Rutherford’s groundbreaking experiment, there were many more experiments that helped refine our understanding of the atom. For example, Niels Bohr built upon Rutherford’s work by proposing a model where electrons occupy specific energy levels around the nucleus. This was a huge leap forward and explained the behavior of atoms in ways that Rutherford’s model couldn’t.
Q: How do we use the knowledge gained from Rutherford’s experiment today?
A: The knowledge gained from Rutherford’s experiment has been used to develop many technologies that we use today. For example, our understanding of the atom has led to the development of nuclear power, nuclear medicine, and other applications. We’ve also been able to use this knowledge to develop new materials and technologies.
Rutherford’s experiment was a crucial turning point in the history of atomic physics. It opened up new avenues for understanding the fundamental building blocks of the universe and paved the way for numerous technological advancements. So next time you think about an atom, remember that tiny gold foil and the brilliant scientist who dared to look beyond the surface.
4.14: Gold Foil Experiment – Chemistry LibreTexts
What is an alpha particle? What did Rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? How did Rutherford explain the observation that most alpha particles went straight through the Chemistry LibreTexts
3.4: Rutherford’s Experiment- The Nuclear Model of the
Describe Rutherford’s gold foil experiment and explain how this experiment altered the “plum pudding” model. The electron was discovered by J.J. Chemistry LibreTexts
Rutherford model | Definition, Description, Image, & Facts
Because only very few of the alpha particles in his beam were scattered by large angles after striking the gold foil while most passed completely through, Rutherford knew that the gold atom’s mass must be Britannica
Discovery of the electron and nucleus (article) | Khan Academy
In his famous gold foil experiment, Rutherford fired a thin beam of α particles (pronounced alpha particles) at a very thin sheet of pure gold. Alpha particles are helium nuclei ( 2 4 Khan Academy
What is the ‘Gold Foil Experiment’? The Geiger-Marsden
Rutherford’s Nobel-winning discovery of α particles formed the basis of the gold foil experiment, which cast doubt on the plum pudding model. Live Science
Rutherford Scattering – MIT
Rutherford Scattering. Edward Jin. MIT Department of Physics. (Dated: April 14, 2022) By scattering α particles produced by 241Am on gold foil, the scattering-cross section per MIT – Massachusetts Institute of Technology
Rutherford scattering – Wikipedia
Hans Geiger, working in Rutherford’s lab, did a series experiments in 1908 showing that alpha particles are “scattered” as they pass through thin layers of mica, and foils of Wikipedia
Rutherford’s gold foil experiment (video) | Khan Academy
If they pass too close to the nucleus of the atoms in the gold foil, their straight path might change because the protons in the nuclei of the gold particles in the gold foil can repel Khan Academy
The Rutherford Scattering Experiment – UC Davis
Ernest Rutherford in 1911, with his postulates concerning the scattering of alpha particles by atoms. Two of his students, Hans Geiger and Ernest Marsden (an undergraduate), ucdavis.edu
See more new information: curtislovellmusic.com
Rutherford’S Gold Foil Experiment – Quick And Simple!
Rutherford Alpha Particle Scattering Experiment ||3D Animated Explanation In Hinglish || Physics12Th
|Rutherford Gold Foil Experiment| | My Inter Academy |
Ruther’S Alpha Scattering Experiment2
Rutherford Gold Foil Experiment – Backstage Science
Discovery Of The Nucleus: Rutherford’S Gold Foil Experiment
The Discovery Of The Atomic Nucleus (3 Of 15)
Rutherford’S Gold Foil Experiment
Link to this article: rutherford’s gold foil experiment using alpha particle scattering concluded that.
See more articles in the same category here: https://curtislovellmusic.com/category/what/